Page 96 - 2023 Taiwan Health and Welfare Report
P. 96
07 2023 Taiwan Health and Welfare Report 07
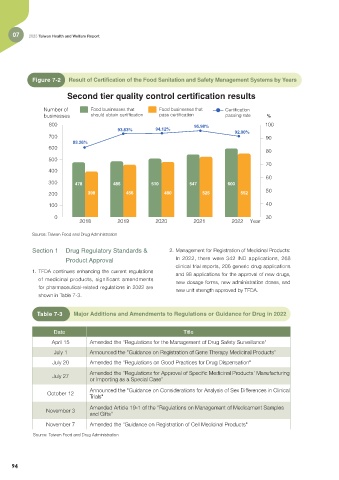
Figure 7-2 Result of Certification of the Food Sanitation and Safety Management Systems by Years
Second tier quality control certification results
Number of Food businesses that Food businesses that Certification
businesses should obtain certification pass certification passing rate %
800 95.98% 100
93.83% 94.12%
92.00%
700 90
83.26%
600
80
500
70
400
60
300 478 486 510 547 600
50
200 398 456 480 525 552
100 40
0 30
2018 2019 2020 2021 2022 Year
Source: Taiwan Food and Drug Administration
Section 1 Drug Regulatory Standards & 2. Management for Registration of Medicinal Products:
Product Approval In 2022, there were 342 IND applications, 268
clinical trial reports, 206 generic drug applications
1. TFDA continues enhancing the current regulations
and 98 applications for the approval of new drugs,
of medicinal products, significant amendments
new dosage forms, new administration doses, and
for pharmaceutical-related regulations in 2022 are
new unit strength approved by TFDA.
shown in Table 7-3.
Table 7-3 Major Additions and Amendments to Regulations or Guidance for Drug in 2022
Date Title
April 15 Amended the "Regulations for the Management of Drug Safety Surveillance"
July 1 Announced the "Guidance on Registration of Gene Therapy Medicinal Products"
July 20 Amended the "Regulations on Good Practices for Drug Dispensation"
Amended the "Regulations for Approval of Specific Medicinal Products' Manufacturing
July 27
or Importing as a Special Case"
Announced the "Guidance on Considerations for Analysis of Sex Differences in Clinical
October 12
Trials"
Amended Article 19-1 of the "Regulations on Management of Medicament Samples
November 3
and Gifts"
November 7 Amended the "Guidance on Registration of Cell Medicinal Products"
Source: Taiwan Food and Drug Administration
94 95