Page 101 - 2023 Taiwan Health and Welfare Report
P. 101
07 Management of Food and Drug 07
4. Fast-track services were available to review 2. By the end of 2022, a total of 371,556 cosmetic
applications to manufacture/import COVID-19 home products completed notification on the Cosmetic
test kits as a special case, and the Ministry actively Products Notification Platform System.
provided consultation and guidance to domestic
Section 3 Quality Surveillance of Medical
manufacturers and importers. By the end of 2022,
Devices and Cosmetics
a total of 12 applications to manufacture COVID-19
home test kits as a special case and 43 applications 1. The surveillance results are shown in Table 7-8.
to import COVID-19 home test kits as a special Unqualified products were disposed according
case were approved, meeting domestic demand. to relevant laws and regulations, and have been
5. TFDA organized the "2022 APEC Medical Devices withdrawn from the shelves.
Regulatory Science Center of Excellence Workshop" 2. TFDA worked with local health bureaus to conduct
to train 53 seed instructors from government, joint inspections. The results are shown in Table 7-9.
industry, and academia in 13 countries and promote Products failing to comply with the requirements
cross-border regulatory harmonization. were disposed of according to relevant laws and
regulations.
Section 2 Source Management of Medical
Devices and Cosmetics Section 4 Safety Management of Medical
Devices and Cosmetics
1. As of the end of 2022, there are 1,331 valid domestic
medical devices manufacturing licenses approved 1. Figure 7-5 shows the number of reported defective
through the Quality Management System (QMS) medical devices and adverse reactions to medical
inspections, and 5,197 valid imported medical devices in Taiwan. TFDA actively monitors 1,427
devices manufacturing licenses approved through the safety alerts related to medical devices from Taiwan
Quality System Documentation (QSD) inspections. and overseas and translated and issued 112 alerts.
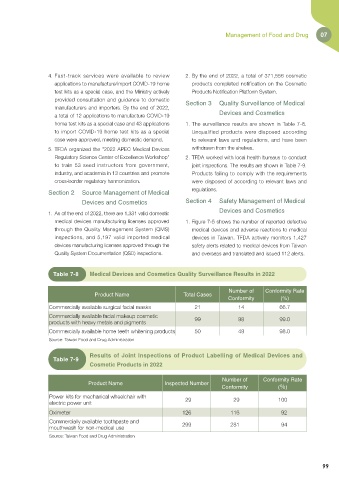
Table 7-8 Medical Devices and Cosmetics Quality Surveillance Results in 2022
Number of Conformity Rate
Product Name Total Cases
Conformity (%)
Commercially available surgical facial masks 21 14 66.7
Commercially available facial makeup cosmetic
products with heavy metals and pigments 99 98 99.0
Commercially available home teeth whitening products 50 49 98.0
Source: Taiwan Food and Drug Administration
Results of Joint Inspections of Product Labelling of Medical Devices and
Table 7-9
Cosmetic Products in 2022
Number of Conformity Rate
Product Name Inspected Number
Conformity (%)
Power kits for mechanical wheelchair with
electric power unit 29 29 100
Oximeter 126 116 92
Commercially available toothpaste and 299 281 94
mouthwash for non-medical use
Source: Taiwan Food and Drug Administration
98 99