Page 102 - 2023 Taiwan Health and Welfare Report
P. 102
07 2023 Taiwan Health and Welfare Report 07
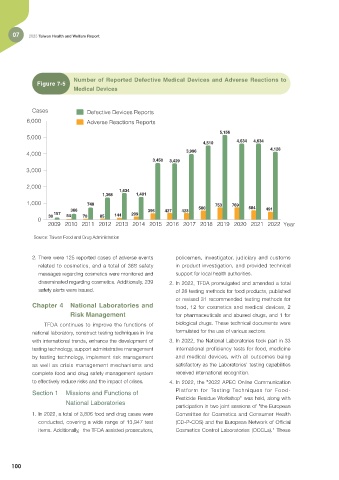
Number of Reported Defective Medical Devices and Adverse Reactions to
Figure 7-5
Medical Devices
Cases Defective Devices Reports
6,000 Adverse Reactions Reports
5,156
5,000
4,510 4,634 4,634
4,128
4,000 3,996
3,450 3,429
3,000
2,000
1,634
1,368 1,401
1,000 748 753 769
580 584
366 395 427 423 491
157 209
30 54 70 85 144
0
2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 Year
Source: Taiwan Food and Drug Administration
2. There were 125 reported cases of adverse events policemen, investigator, judiciary and customs
related to cosmetics, and a total of 388 safety in product investigation, and provided technical
messages regarding cosmetics were monitored and support for local health authorities.
disseminated regarding cosmetics. Additionally, 239 2. In 2022, TFDA promulgated and amended a total
safety alerts were issued. of 28 testing methods for food products, published
or revised 31 recommended testing methods for
Chapter 4 National Laboratories and food, 12 for cosmetics and medical devices, 2
Risk Management for pharmaceuticals and abused drugs, and 1 for
TFDA continues to improve the functions of biological drugs. These technical documents were
national laboratory, construct testing techniques in line formulated for the use of various sectors.
with international trends, enhance the development of 3. In 2022, the National Laboratories took part in 33
testing technology, support administrative management international proficiency tests for food, medicine
by testing technology, implement risk management and medical devices, with all outcomes being
as well as crisis management mechanisms and satisfactory as the Laboratories' testing capabilities
complete food and drug safety management system received international recognition.
to effectively reduce risks and the impact of crises. 4. In 2022, the "2022 APEC Online Communication
Platform for Testing Techniques for Food-
Section 1 Missions and Functions of
Pesticide Residue Workshop" was held, along with
National Laboratories
participation in two joint sessions of "the European
1. In 2022, a total of 3,806 food and drug cases were Committee for Cosmetics and Consumer Health
conducted, covering a wide range of 13,947 test (CD-P-COS) and the European Network of Official
items. Additionally, the TFDA assisted prosecutors, Cosmetics Control Laboratories (OCCLs)." These
100 101