Page 93 - 2023 Taiwan Health and Welfare Report
P. 93
Management of Food and Drug 07
Taiwan Food and Drug Administration (hereafter Section 1 Food Regulatory Standards
referred to as TFDA) spares no efforts in workings and Product Reviews and
to protect the health of consumers. To achieve this
Registration
core value, the key working points of the agency in
2022 focus on: bolstering legal standards and register 1. Regulatory Standards
mechanisms; solidifying food businesses supervisions; In 2022, 34 revisions were made to regulations
establishing a detailed supply chain monitoring system; in order to enhance the food management
improving national laboratory capacity and capability; standards in our country. These revisions included
setting up risk precautionary and management the establishment of the "Food Types and Their
mechanisms; and proactively bolstering public Produced or Manufactured Areas from Japan
communications and advocates, so as to provide an Whose Imports are Suspended" the implementation
environment ensuring drug safety and effectiveness, as measures for the "Regulations for the Security and
well as food safety and health to our consumers. the Maintenance of Personal Information Files in
Food Businesses," and "Imported Livestock and
Chapter 1 Management of Food Poultry Meat Products Shall Be Accompanied
by Official Certificates Issued by the Competent
TFDA continues to implement its "Five-Point
Authority of the Exporting Country." These revisions
Food Safety" policy to achieve inter-domain integration
were implemented to strengthen the regulatory
of five major aspects: source control management,
framework for food management in our country.
production management, market inspection,
manufacturers and vendors liability, and supervision 2. Product Reviews and Registration
by the citizens in order to create a comprehensive In 2022, TFDA registered the specific foods and
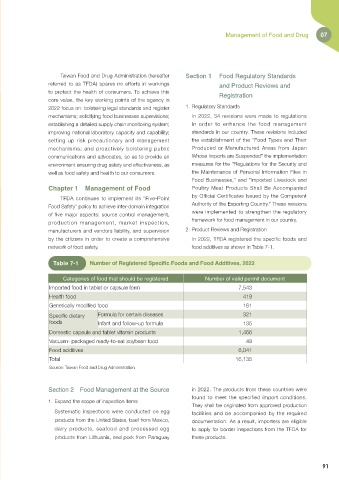
network of food safety. food additives as shown in Table 7-1.
Table 7-1 Number of Registered Specific Foods and Food Additives, 2022
Categories of food that should be registered Number of valid permit document
Imported food in tablet or capsule form 7,543
Health food 419
Genetically modified food 161
Specific dietary Formula for certain diseases 321
foods Infant and follow-up formula 135
Domestic capsule and tablet vitamin products 1,466
Vacuum- packaged ready-to-eat soybean food 49
Food additives 6,041
Total 16,135
Source: Taiwan Food and Drug Administration
Section 2 Food Management at the Source in 2022. The products from these countries were
found to meet the specified import conditions.
1. Expand the scope of inspection items
They shall be originated from approved production
Systematic inspections were conducted on egg facilities and be accompanied by the required
products from the United States, beef from Mexico, documentation. As a result, importers are eligible
dairy products, seafood and processed egg to apply for border inspections from the TFDA for
products from Lithuania, and pork from Paraguay these products.
91