Page 154 - 2023 Taiwan Health and Welfare Report
P. 154
12 2023 Taiwan Health and Welfare Report 12
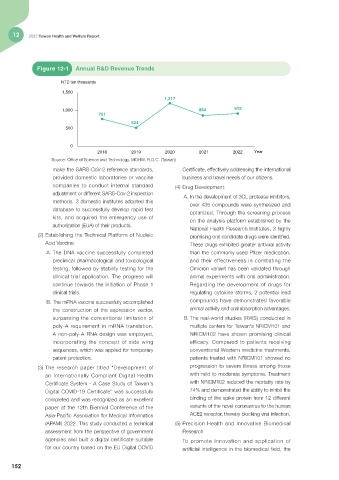
Figure 12-1 Annual R&D Revenue Trends
NTD ten thousands
1,500
1,217
1,000 864 912
751
524
500
0
2018 2019 2020 2021 2022 Year
Source: Office of Science and Technology, MOHW, R.O.C. (Taiwan)
make the SARS-CoV-2 reference standards, Certificate, effectively addressing the international
provided domestic laboratories or vaccine business and travel needs of our citizens.
companies to conduct internal standard (4) Drug Development:
adjustment or different SARS-Cov-2 inspection
A. In the development of 3CL protease inhibitors,
methods. 3 domestic institutes adopted this
over 435 compounds were synthesized and
database to successfully develop rapid test
optimized. Through the screening process
kits, and acquired the emergency use of
on the analysis platform established by the
authorization (EUA) of their products.
National Health Research Institutes, 3 highly
(2) Establishing the Technical Platform of Nucleic promising oral candidate drugs were identified.
Acid Vaccine These drugs exhibited greater antiviral activity
A. The DNA vaccine successfully completed than the commonly used Pfizer medication,
preclinical pharmacological and toxicological and their effectiveness in combating the
testing, followed by stability testing for the Omicron variant has been validated through
clinical trial application. The progress will animal experiments with oral administration.
continue towards the initiation of Phase 1 Regarding the development of drugs for
clinical trials. regulating cytokine storms, 2 potential lead
B. The mRNA vaccine successfully accomplished compounds have demonstrated favorable
the construction of the expression vector, animal activity and oral absorption advantages.
surpassing the conventional limitation of B. The real-world studies (RWS) conducted in
poly-A requirement in mRNA translation. multiple centers for Taiwan's NRICM101 and
A non-poly-A RNA design was employed, NRICM102 have shown promising clinical
incorporating the concept of side wing efficacy. Compared to patients receiving
sequences, which was applied for temporary conventional Western medicine treatments,
patent protection. patients treated with NRICM101 showed no
(3) The research paper titled "Development of progression to severe illness among those
an Internationally Compliant Digital Health with mild to moderate symptoms. Treatment
Certificate System - A Case Study of Taiwan's with NRICM102 reduced the mortality rate by
Digital COVID-19 Certificate" was successfully 74% and demonstrated the ability to inhibit the
completed and was recognized as an excellent binding of the spike protein from 12 different
paper at the 12th Biennial Conference of the variants of the novel coronavirus to the human
Asia-Pacific Association for Medical Informatics ACE2 receptor, thereby blocking viral infection.
(APAMI) 2022. This study conducted a technical (5) Precision Health and Innovative Biomedical
assessment from the perspective of government Research
agencies and built a digital certificate suitable To promote innovation and application of
for our country based on the EU Digital COVID artificial intelligence in the biomedical field, the
152 153