Page 98 - 2024 Taiwan Health and Welfare Report
P. 98
2024 Taiwan Health and Welfare Report
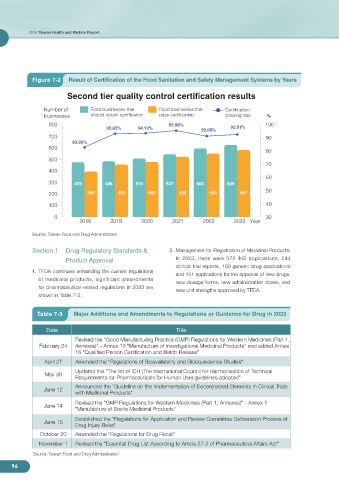
Figure 7-2 Result of Certification of the Food Sanitation and Safety Management Systems by Years
Second tier quality control certification results
Number of Food businesses that Food businesses that Certification
businesses should obtain certification pass certification passing rate %
800 95.98% 100
93.83% 94.12% 92.81%
92.00%
700 90
83.26%
600
80
500
70
400
60
300 478 486 510 547 600 626
50
200 398 456 480 525 552 581
100 40
0 30
2018 2019 2020 2021 2022 2023 Year
Source: Taiwan Food and Drug Administration
Section 1 Drug Regulatory Standards & 2. Management for Registration of Medicinal Products:
In 2023, there were 372 IND applications, 244
Product Approval
clinical trial reports, 180 generic drug applications
1. TFDA continues enhancing the current regulations and 101 applications for the approval of new drugs,
of medicinal products, significant amendments new dosage forms, new administration doses, and
for pharmaceutical-related regulations in 2023 are new unit strengths approved by TFDA.
shown in Table 7-3.
Table 7-3 Major Additions and Amendments to Regulations or Guidance for Drug in 2023
Date Title
Revised the "Good Manufacturing Practice (GMP) Regulations for Western Medicines (Part 1,
February 24 Annexes)" - Annex 13 "Manufacture of Investigational Medicinal Products" and added Annex
16 "Qualified Person Certification and Batch Release"
April 27 Amended the "Regulations of Bioavailability and Bioequivalence Studies"
Updated the "The list of ICH (The International Council for Harmonisation of Technical
May 30 Requirements for Pharmaceuticals for Human Use) guidelines adopted"
June 12 Announced the "Guideline on the Implementation of Decentralized Elements in Clinical Trials
with Medicinal Products"
Revised the "GMP Regulations for Western Medicines (Part 1, Annexes)" - Annex 1
June 14 "Manufacture of Sterile Medicinal Products"
June 15 Established the "Regulations for Application and Review Committee Deliberation Process of
Drug Injury Relief"
October 20 Amended the "Regulations for Drug Recall"
November 1 Revised the "Essential Drug List According to Article 27-2 of Pharmaceutical Affairs Act"
Source: Taiwan Food and Drug Administration
96