Page 103 - 2024 Taiwan Health and Welfare Report
P. 103
07 | Management of Food and Drug
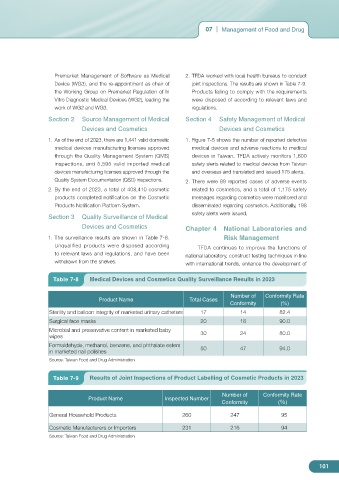
Premarket Management of Software as Medical 2. TFDA worked with local health bureaus to conduct
Device (WG3), and the re-appointment as chair of joint inspections. The results are shown in Table 7-9.
the Working Group on Premarket Regulation of In Products failing to comply with the requirements
Vitro Diagnostic Medical Devices (WG2), leading the were disposed of according to relevant laws and
work of WG2 and WG3. regulations.
Section 2 Source Management of Medical Section 4 Safety Management of Medical
Devices and Cosmetics Devices and Cosmetics
1. As of the end of 2023, there are 1,441 valid domestic 1. Figure 7-5 shows the number of reported defective
medical devices manufacturing licenses approved medical devices and adverse reactions to medical
through the Quality Management System (QMS) devices in Taiwan. TFDA actively monitors 1,600
inspections, and 5,595 valid imported medical safety alerts related to medical devices from Taiwan
devices manufacturing licenses approved through the and overseas and translated and issued 175 alerts.
Quality System Documentation (QSD) inspections. 2. There were 99 reported cases of adverse events
2. By the end of 2023, a total of 408,410 cosmetic related to cosmetics, and a total of 1,175 safety
products completed notification on the Cosmetic messages regarding cosmetics were monitored and
Products Notification Platform System. disseminated regarding cosmetics. Additionally, 198
safety alerts were issued.
Section 3 Quality Surveillance of Medical
Devices and Cosmetics Chapter 4 National Laboratories and
1. The surveillance results are shown in Table 7-8. Risk Management
Unqualified products were disposed according TFDA continues to improve the functions of
to relevant laws and regulations, and have been national laboratory, construct testing techniques in line
withdrawn from the shelves. with international trends, enhance the development of
Table 7-8 Medical Devices and Cosmetics Quality Surveillance Results in 2023
Number of Conformity Rate
Product Name Total Cases
Conformity (%)
Sterility and balloon integrity of marketed urinary catheters 17 14 82.4
Surgical face masks 20 18 90.0
Microbial and preservative content in marketed baby 30 24 80.0
wipes
Formaldehyde, methanol, benzene, and phthalate esters 50 47 94.0
in marketed nail polishes
Source: Taiwan Food and Drug Administration
Table 7-9 Results of Joint Inspections of Product Labelling of Cosmetic Products in 2023
Number of Conformity Rate
Product Name Inspected Number
Conformity (%)
General Household Products 260 247 95
Cosmetic Manufacturers or Importers 231 216 94
Source: Taiwan Food and Drug Administration
101