Page 104 - 2024 Taiwan Health and Welfare Report
P. 104
2024 Taiwan Health and Welfare Report
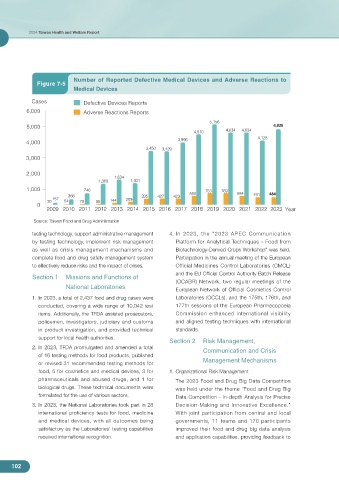
Number of Reported Defective Medical Devices and Adverse Reactions to
Figure 7-5
Medical Devices
Cases Defective Devices Reports
6,000 Adverse Reactions Reports
5,156
5,000 4,826
4,510 4,634 4,634
4,128
3,996
4,000
3,450 3,429
3,000
2,000
1,634
1,368 1,401
1,000 748 753 769
580 584 484
366 395 427 423 491
157 209
30 54 70 85 144
0
2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 Year
Source: Taiwan Food and Drug Administration
testing technology, support administrative management 4. In 2023, the "2023 APEC Communication
by testing technology, implement risk management Platform for Analytical Techniques - Food from
as well as crisis management mechanisms and Biotechnology-Derived Crops Workshop" was held.
complete food and drug safety management system Participation in the annual meeting of the European
to effectively reduce risks and the impact of crises. Official Medicines Control Laboratories (OMCL)
and the EU Official Control Authority Batch Release
Section 1 Missions and Functions of
(OCABR) Network, two regular meetings of the
National Laboratories European Network of Official Cosmetics Control
1. In 2023, a total of 2,437 food and drug cases were Laboratories (OCCLs), and the 175th, 176th, and
conducted, covering a wide range of 10,042 test 177th sessions of the European Pharmacopoeia
items. Additionally, the TFDA assisted prosecutors, Commission enhanced international visibility
policemen, investigators, judiciary and customs and aligned testing techniques with international
in product investigation, and provided technical standards.
support for local health authorities.
Section 2 Risk Management,
2. In 2023, TFDA promulgated and amended a total
of 16 testing methods for food products, published Communication and Crisis
or revised 31 recommended testing methods for Management Mechanisms
food, 5 for cosmetics and medical devices, 3 for 1. Organizational Risk Management
pharmaceuticals and abused drugs, and 1 for The 2023 Food and Drug Big Data Competition
biological drugs. These technical documents were was held under the theme "Food and Drug Big
formulated for the use of various sectors. Data Competition - In-depth Analysis for Precise
3. In 2023, the National Laboratories took part in 28 Decision-Making and Innovative Excellence."
international proficiency tests for food, medicine With joint participation from central and local
and medical devices, with all outcomes being governments, 11 teams and 170 participants
satisfactory as the Laboratories' testing capabilities improved their food and drug big data analysis
received international recognition. and application capabilities, providing feedback to
102